

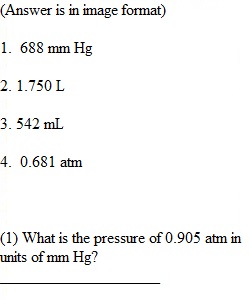
Q Gases Assignment Print Name_____________________________ Date_______________________ Directions: read each question carefully and select the correct answer. Provide all mathematical work in order to receive credit. You may use additional paper to show work-out calculations. (1) What is the pressure of 0.905 atm in units of mm Hg? ____________________ (2) The initial volume of a gas cylinder is 750.0 mL. If the pressure of a gas inside the cylinder changes from 840.0 mm to 360.0 mm Hg, what is the final volume (in Liters) the gas occupies? __________________________ (3) What is the final volume (in mL) of a balloon that was initially 500.0 mL at 25 °C and was then heated to 50 °C ? ____________________ (4) A sample of helium gas initially at 37.0 °C, 785 torr and 2.00 L was heated to 58.0 °C while the volume expanded to 3.24 L. What is the final pressure in atm? ___________________________ (5) How many moles of gas were added to a balloon that started with 2.3 moles of gas and a volume of 1.4 L given that the final volume was 7.2 L?______________________________ (6) If a mixture of gases contained 78% nitrogen at a pressure of 984 torr and 22% carbon dioxide at 345 torr, what is the total pressure of the system? ___________________
View Related Questions